How to Manage, Monetize, and Secure IoT Medical Devices
Best Practices for Medical Device Manufacturers
Protect Your Devices to Protect Your Patients
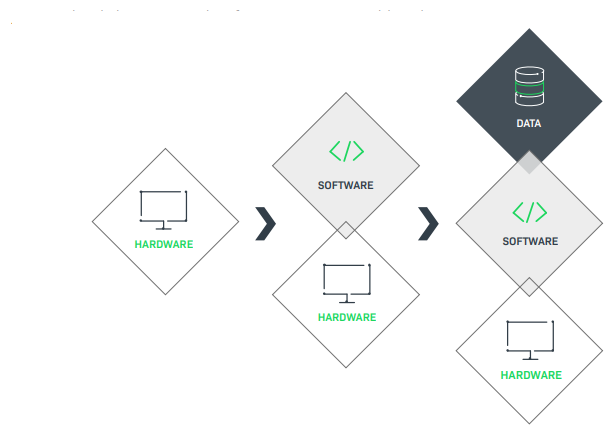
The quality of patient care is central to healthcare. In the fast moving world of medical devices, software is at the heart of innovation, with producers shifting from hardware-focused to subscription-based business models. Today embedded software is integral to these medical devices. It‘s critical that device manufacturers prescribe success by having appropriate processes and systems in place.
Medical device companies need business solutions that:
- Deliver a secure product.
- Manage software and streamline updates.
- Increase the profitability of devices.
Healthcare Security is Business Critical
The Internet of Things (IoT) is changing the way technology is consumed, driving a transformation in the healthcare industry. These are part of what VDC Research highlights as the “changing device functionality requirements” impacting traditional embedded or industrial markets. Healthcare and medical device manufacturing are addressing compliance, cost pressures, consolidation, continuous management, commoditization, and business model changes.
The industry’s transformation and the shift to IoT medical devices represent:
- A focus on value-based healthcare, improving clinical outcomes while lowering overall costs.
- Incorporation of technology in the diagnostic and post-procedural phase to improve value across the continuum of care.
- Reliance on technology services and insights to deliver operational efficiencies and better patient care management.
- A need to secure IoT devices to ensure increased profitability.
- Use of real-time insights to deliver personalized patient care.
Medical Device Value Shift
Innovate and Differentiate
As medical devices evolve from conventional hardware to software-enabled systems that capture valuable data, a robust security, delivery, and updates strategy is essential.
Tip
Device manufacturers are responsible for everything they distribute or host. You must have the tools and processes in place to identify, mitigate, and remediate security flaws.
The Medical Industry’s Specific Challenges & Needs
The medical industry now faces unique challenges in protecting their software supply chain. While undocumented open source code is in virtually all software, unique precautions apply in healthcare, where HIPAA requires device manufacturers to minimize the risk of shipping products to customers with unpatched vulnerabilities. Specific needs in this field have often meant that:
- More complex devices require compatibility or dependency checks before a software update,
- Technicians have needed to manually verify hardware compatibilities before starting updates, and
- There was no visibility or insight into software or firmware versions on devices.
A better approach is possible. Autonomous updates can replace costly, time-consuming manual processes. This allows the supplier or device manufacturer to be prepared for regulatory compliance, with a complete track record of what software is running where. This is particularly necessary as the frequency and sophistication of security exploits increases.
The Medical Industry Has an Action Plan. Do You?
The medical industry has detailed an action plan to secure medical devices, with clearly defined responsibilities for manufacturers. Medical cybersecurity regulations emphasize managing cybersecurity risks throughout a medical device’s entire lifecycle.
The U.S. Food & Drug Administration has a Medical Device Safety Action Plan, with the goals of reducing attack surfaces, controlling access to software and data, and keeping software and firmware up to date. The FDA’s cyber regulations are primarily focused on medical devices with cybersecurity risks (networked, containing software, etc.).
- Medical device manufacturers must build the capability to patch device security into a product’s design and to provide appropriate data regarding this capability to the FDA as part of the device’s pre-market submission to demonstrate reasonable assurance cybersecurity procedures and testing (including SBOMs).
- Post-market requirements include the need to monitor, identify, and address cybersecurity vulnerabilities and exploits; this relies on maintaining SBOMs as part of an SCA program.
Similarly, the EU Medical Device Regulation (MDR) applies to manufacturers, authorized representatives, importers or distributors of medical devices in the EU. These parties must identify vulnerabilities and potential exploits in their devices; design, develop, and maintain medical devices with robust cybersecurity features, and provide timely software updates and security patches.
A Closer Look at SBOMs
A software bill of materials is a formal and queryable record containing the details and relationships of various components used in building software. Think of it as an ingredients label for your software application.
The multiple uses of SBOMs include automating the inventory processes for open source software and third-party components, enabling transparency for customers and authorities, and tracking vulnerabilities for the components in use. Taken together, these allow device manufacturers to understand the risk present in their devices and act accordingly to secure them.
Medical device manufacturers can use SBOMs to address five critical questions to stay in control of OSS usage:
- Are we exposed to a specific vulnerability?
- Are our components up to date?
- Where is the risk and how do we mitigate it?
- Do we know what components are in our applications?
- Do we have any legal and/or security compliance issues per our policy?
SBOM
A Software Bill of Materials (SBOM) is a formal and queryable record containing the details and relationships of various components used in building software
WHAT GETS SCANNED
- EXECUTABLES
- COMMERCIAL LIBRARIES
- PROPRIETARY SOFTWARE
- OSS COMPONENTS
INFORMATION RECORDED
- SUPPLIER INFORMATION
- SOFTWARE COMPONENTS + VERSIONS
- DEPENDENCIES
- AUTHOR NAMES
- LICENSES
- SECURITY VULNERABILITIES
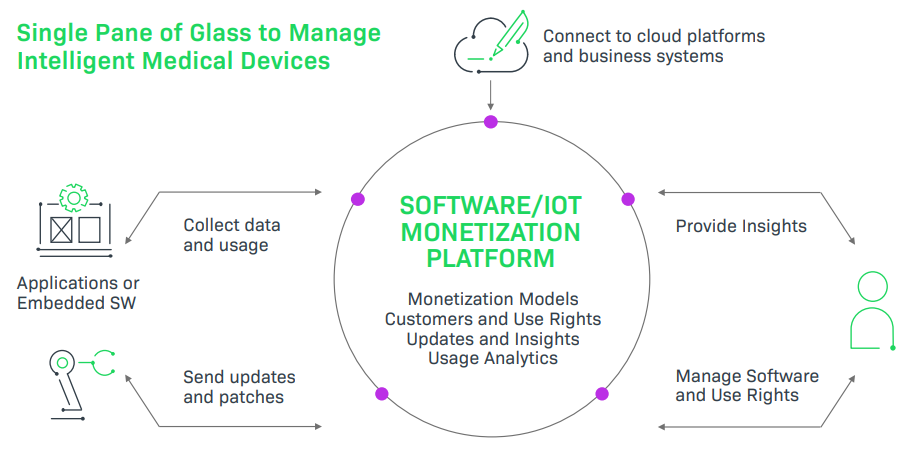
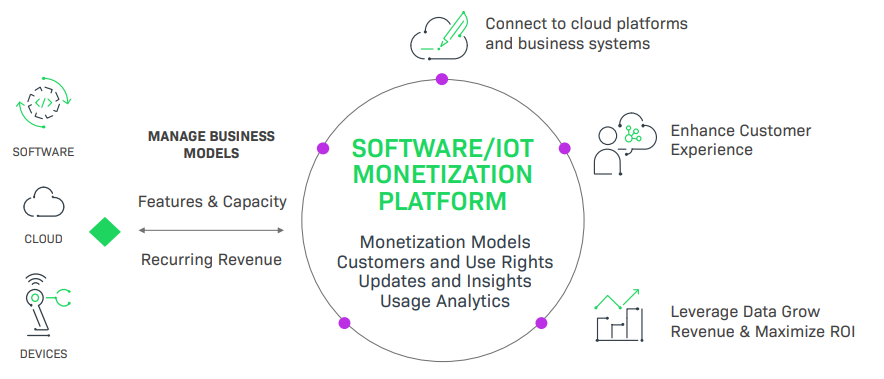
Monetization Opportunities for the Medical Industry
The pathway to a modern, secure, and profitable business model for medical devices centers on helping customers stay current and secure, knowing what customers are using, and learning from software and device insights. To achieve this, medical device manufacturers must evaluate how to implement new business models, grow recurring revenue, stay in compliance with industry regulations, and ship secure software products that are free of vulnerabilities.
Depending on the application and the industry, updates may need to be delivered quarterly, monthly, weekly, or even continuously. The process of managing software updates needs to scale. Manual processes will break, particularly as the number of devices (including tablets and sensitive machines) grows.
An automated, comprehensive IoT monetization platform:
- Securely and accurately provides entitlement-driven delivery of updates and security patches,
- Increases security and vulnerability mitigation with an end-to-end process,
- Stops revenue leakage from updates delivered to non-eligible customers, thereby protecting intellectual property,
- Implements end-to-end process automation based on subscriptions and other entitlement information,
- Offers the usage data and analytics to help businesses grow, and
- Helps medical device manufacturers offer the right monetization models for the right products at the right price.
Dynamic & Agile Business Transformation Unlock New Business Models
Keep your Customers Front and Center.
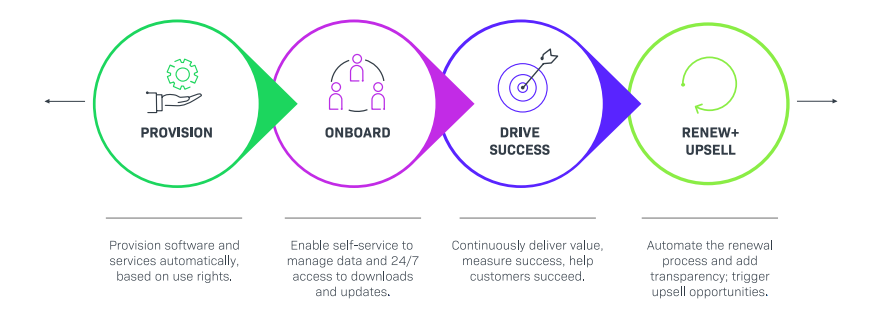
Keep your customers—and their patients—front and center.
Medical device manufacturers should turn to software monetization and software composition analysis solutions that enable implementation of new business models, grow recurring revenue, stay in compliance with FDA/MDR regulations and ship secure software products that are free of vulnerabilities. Adhering to industry best practices—operating within a security framework, developing and maintaining an OSS policy, and generating SBOMs—can help drive digital transformation and meet industry requirements efficiently. The end result is that your code, your customers, and your reputation all remain healthy.
Resources
Webinar
6 Steps to Launching Usage-Based Pricing for SaaS and AI
Tuesday, February 17, 2026
In this webinar, Nicole Segerer, General Manager at Revenera, will share this practical 6-step framework for launching usage-based pricing in SaaS, AI, and even on-premises products.
Webinar
Software License Compliance Best Practices
Wednesday, February 25, 2026
Join our quarterly webinar series, "Software License Compliance Best Practices," where we'll explore the latest strategies and methodologies for ensuring compliance and addressing piracy and overuse.
Report
AI Monetization, Done Right: A Practical Guide for Product and Pricing Leaders
This guide outlines how product and pricing leaders can achieve sustainable AI profitability by aligning pricing, usage, and cost structures.
In-person Event
Revenera Connect 2026: San Jose
Thursday, March 19, 2026
Register for Revenera Connect 2026 in San Jose! The event will offer new insights and expertise into the latest monetization and pricing trends, practices and technologies and provide you with new ideas for how to make your business more successful in 2026.
Webinar
SoftSummit 2026
April 28, 29 & 30 (Tuesday, Wednesday & Thursday)
Report
2026 Frost & Sullivan Product Leadership Recognition Report
This exclusive report reveals how Revenera is shaping the future of software monetization, compliance, and lifecycle management.
Want to learn more?
See how Revenera's Software Monetization platform can help you take products to market fast, unlock the value of your IP and accelerate revenue growth.